Invisible UV light plays a very important role in nature and industry. It can also be used as a tool for beautiful STEM experiments.
We are mostly blind
Human vision is covering quite a small range of electromagnetic spectrum, i.e., colors of light. By light we mean everything from dangerous gamma rays and X-rays to body heat (infrared) and radio waves. Our eyes evolved to take advantage of the Sun’s light, but they are also limited by the biochemistry that has been available for evolution to work with.
This biochemical evolution yielded three types of color sensitive receptors that together can detect rainbow colors covering wavelengths from about 380 (violet-blue side of rainbow) to about 760 nanometers (red colors). To everything else, we are blind.
Interestingly enough, some animals evolved vision that goes beyond what humans can see. For example, very important, but invisible to humans, are colors in the ultraviolet (UV) range. Those are colors that extend the violet part of the rainbow even further to shorter wavelengths, all the way down to 10 nanometers (nm), where X-rays start. The UV spectrum is subdivided into standardized “UV colors”, where the UV-A (or “soft UV” or “near UV”) between about 300 and 400 nm is of our interest here.
Although soft UV from the Sun is all around us every day, a direct exposure of eye to the UV light should be avoided as it can damage retina. Even shorter UV wavelengths are a serious health risk for eyes and skin. Fortunately, the Earth’s atmosphere mostly absorbs the most dangerous UV light from the Sun.
This makes the Earth’s surface illuminated with the solar UV-A light, which has been an evolutionary niche for flowers and their pollinators. Some flowers create patterns in the reflected UV to attract pollinators and guide them to the nectar and pollen. These UV markings are visible to insects, while humans do not see them.
There are some other animals that can see the UV-A as this helps them in finding food. Recently, research showed that young people actually can see bright UV light, but this is a serious health risk for eyes and should not be practiced.
Black is the color of light
Since UV light is more energetic than ordinary visible colors, many types of molecules interact with the UV colors in an interesting way. They can absorb the invisible UV light and then immediately release this energy as visible light. This phenomenon is called fluorescence. It can also happen in some material with visible light being absorbed, but with the UV it is more spectacular because we can make stuff glow in the dark.

The UV-A lamps needed for this type of experiments are easily available in electronics stores or on the Internet. They are sometimes called “black light”. Make sure that the lamp emits wavelengths longer than 300 nm as this will require only UV protection goggles for safety. There are several types of devices that produce such black light, and the most easily available are tubes and LEDs. Together with an UV-A lamp, purchase UV protection safety goggles. This is very important, especially if you show this experiment to small kids who will sooner or later point these lights directly into their eyes.
Once you have UV light source and UV protection goggles, the fun can start. The first thing is to explore the immediate world around you. Turn off the lights and go around your house using only the black light. Fluorescence will make many ordinary house items glow in the dark. Various types of plastics, paper (because modern paper contains fluorescent dye that makes it appear whiter), dust flakes (this will show how dusty your home is), liquid detergent (it also contains fluorescent compounds that make clothes look brighter), oils, tonic water, your fingernails, etc. Warning – be prepared for shocking scenes on your kitchen stove and around bathroom sink and toilet.
More biology-oriented fluorescence examples can be found when illuminating plants and animals (we can call this biofluorescence). Flowers can have amazing fluorescent colors, while a recent discovery revealed that ripe bananas glow bright blue, probably to attract animals that eat them. It has been known for a long time that scorpions glow under black light, but it is still debated what molecule (or probably molecules) is the source of this glow and what biological purpose it serves. In recent years, scientists have been discovering that some unexpected animals have fluorescent properties, such as platypus, dotted tree frog, flying squirrel, and many more.
Turning natural green into bloody red
One experiment that you should try with your kids is chlorophyll fluorescence. Chlorophyll is a pigment that gives plants ability to perform photosynthesis, which transforms energy from light into chemical energy used as food by plants. Chlorophyll fluorescence is an important method in measuring photosynthesis.
The experiment is simple as it takes a few simple steps:
Pick some green plants (I used ordinary grass and it worked) and put them into a jar
Add isopropyl alcohol and start mashing the plants (this will speed up breaking of the plant cells and squeeze chlorophyll out of them).
When the alcohol becomes green, filter it through a coffee filter to extract pure green liquid.
Illuminate this green liquid with UV and it will glow bright red.

Mesmerizing colors
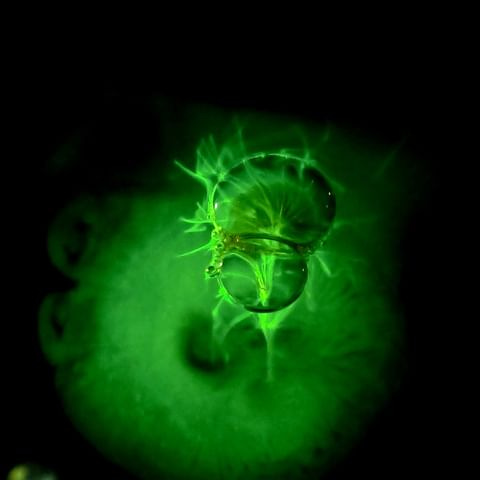
A sure way how to capture your kids’ attention with fluorescence is to give them opportunity to play with a fluorescent dye. Uranine (or D&C Yellow no. 8; the disodium salt form of fluorescein) is a molecule that produces intense yellow-green fluorescence when dissolved in a liquid (e.g., in water). It gets excited by blue colors, but also by the UV-A light, which creates a spectacular glow in the dark. While we can’t see the UV light illuminating the liquid, even the tiniest amounts of uranin exhibit a bright glow.
Uranin is easily available in ordinary online stores, and it is harmless and washable. It is widely used as a tracer for leak detection or water flow path. However, it is an intense dye, so you probably do not want to test its ability to stain your skin or clothes.

Instructions for this experiment are simple:
Dissolve a tiny amount of uranin in some non-water liquid (soap, milk, …)
Fill a glass container or jar with water, darken the room, and shine UV light onto the water
Drop small volumes of the uranin solution into the water (pipette helps with this) and observe the features created by the glowing liquid
Doing this in a complete darkness is the most impressive. Be creative in what to mix and how to mix – uranin will trace microfluid flows that can look like they are out of this world! Sometimes a small change of the liquid properties can result in beautiful, unexpected glowing features.