The underappreciated world of surface tension
… and often repeated simple explanations that are not so simple after all
Most people have heard of surface tension force, together with some general explanations of what it is. However, the science of surface tension is quite complicated, but also beautiful and fun to explore together with your kids.
The angels are in details, not the devil
A really good educational experiment is the one that leaves more questions opened than answered. This means that we first need to ask lots of questions, which is typically not the case because we tend to ignore details and focus only at the one big question behind a presented experiment. This is a problem for two reasons.
First, this leads to misconception that exploring nature is something straightforward, mundane, and only for geeks who are weird anyway. “Oh, not yet another boring science experiment”, students often say as they expect a step-by-step cookbook to follow. Unfortunately, when kids reach mid-teenage years their attitudes toward STEM topics are mostly set. Their love for STEM has to be imprinted when they are younger. The fact that the interest of 9-12-years-old kids for STEM is dropping is very worrisome. This declining trend correlates with the increasing societal development, but we will cover that issue separately in one of the future texts.
Second, noticing details is an important skill. Whatever the profession, there are benefits from being able to notice details that can open a door to new information or insights. Presenting oversimplistic descriptions of the nature behind an experiment misses the main point – the experiment’s seemingly simple explanation stands on a mountain of complex details. In other words, if you tell kids that a car is moving just because the engine is running, don’t be surprised if the kids end up oblivious to the complexity and beauty of the engine’s science and technology.
Fortunately, most experiments are packed with details, but the problem is that we do not pay enough attention to them either because we do not see them, or we are afraid that details will consume all the time we have for the experiment. The end result is that kids are not given a chance to think about noticing the details and to wonder about them.
A strange microcosmos of liquid surfaces
I started with this long introduction because one of the underappreciated topics in science education is the microphysics and chemistry of surface tension effects. To be honest, the entire field of fluid dynamics is not used enough in education, but the sub-field of surface tension carries an extra beauty, I think. The key element here is that this beauty is hidden in countless details that we experience daily but are essentially oblivious to them.
Surface tension phenomena are a very prolific field of research and very important in industry. By “tension” we mean electrostatic forces that molecules impose onto each other (in some special cases also magnetic forces, but in water it is the electrostatic hydrogen bond that dominates between molecules). Every molecule interacts with its neighbors on every side by pulling each other in an endless game of tug of war. Since an average molecule is in average surrounded by the same number of other molecules in all directions, the net result is an average standstill. Except at the surface, where the surface molecules experience the electrostatic tug of war only toward one side. This transforms the fluid surface into a very strange microcosmos where unusual phenomena can happen. The surface tug of war can be measured as a surface tension force that keeps the surface molecules attached to the rest of the liquid.

But surface tension plays one additional interesting game. If a part of the surface is curved, the surface tension will try to flatten it: molecules sticking out will be pulled back toward the bulk of the liquid, and any surface depression will be refilled by molecules pulled in from the surface. An overall balance is possible only if the surface is flat, where on average each surface molecule is under a balanced tug of war with other surface molecules.

The only thing missing now in this picture is – the end points as the surface must end somewhere. The final surface shape is an interesting mathematical problem – the minimum surface for a given volume of liquid. The surface is minimized because the surface molecules are pulled into the liquid interior, which means that the liquid keeps sucking in surface molecules as long as there is excess surface. We will come back to this in future episodes, including the amazing story of shapes of soap films.
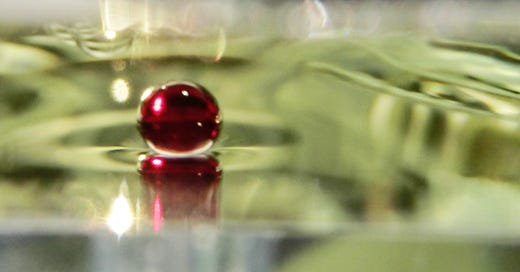
However, there is one special situation: what if there is no wall to touch at all? Well, the only thing that the surface can do is to close on itself. This is the case in raindrops and liquids floating on another liquid. In such a case it is impossible to create an overall flat surface. Hence, the surface forces will move molecules until each segment of the surface feels the same distribution of forces. This results in a sphere (or circle in case of floating liquids). Any bump will be pulled back into the sphere, any dent will be refilled. Notice that this is also the minimal surface for a given volume of liquid.

Indeed, small raindrops are spheres, not teardrops (while larger ones are flattened by air drag). If you have a slow-motion camera, you can take a video of a falling water droplet and convince yourself. In the same way, oil floating on water forms a circle, and water drops within oil are spheres, etc. Often this process of surface reshaping is slow enough to observe its evolution from irregular shapes into a circle.


Of course, if you feel that all this is a simplified picture of surfaces, you are on the right track. The surface molecules are under a never-ending process of fluctuations that depends on many factors. Even for the most common surface we know – that of water – it is still not entirely clear how molecules manage to detach from liquid into vapor. We also have not mentioned additional surface tension forces due to interactions between the fluid molecules and molecules of other surfaces that the fluid is touching.
There are many directions we can take with all this, from insects walking on water to trees sucking water 100 meters high. But kids are typically introduced to the physics (and chemistry) of surface tension through two experiments: soap bubbles (that we will discuss in future episodes) and pepper and soap experiment. The latter is what we will explore in the next episode, together with a beautiful phenomenon of Marangoni bursting.