Secret life of drying droplets
A drying droplet is easy to prepare, but not so easy to explain in detail. This makes it an ideal STEM experiment of tunable complexity.
Not all stains are created equal
It is a long stretch from a coffee stain on your table and limescale deposits on your bathroom walls to purification of pharmaceuticals, de-icing of airplanes, inkjet printing, or manufacturing of novel electronic materials. But even the longest journeys begin with a single step, and in this STEM journey, the first step is indeed with a coffee stain.
In 1997 a group of researchers published a study in the prestigious journal Nature that explained the commonly observed phenomenon of coffee stains. You might have noticed that drops of coffee (or some similar colored liquid) produce brown rings as a residue instead of uniform spots. A ring seems illogical because we expect the drop to shrink while drying, leaving the colored particles smoothly behind during the process. This study, for the first time, identified a sequence of phenomena in a small drop of drying liquid that produce a ring stain.
This work inspired many other scientists to explore this type of phenomena in more detail. Their motivation has come from different areas of applications – from engineering problems of paint deposition or medical quests for cost-effective diagnosis techniques to pharmaceutical, chemical and electronics industries attempting to control creation of their products on a nanoscale level. In today’s STEM story we open the door to this world of physics and chemistry of drying droplets. Once you open this door, you can make journeys in many different directions (including experiments based on the content of your liquor cabinet - for learning STEM, of course).
The main part of this STEM experiment is very simple:
take some colored liquid or dissolve some household chemical in distilled water
put a few drops of different sizes on a clean glass surface
also make a few drops of distilled water as a control (those should not leave any residue)
let it dry and, once its dries out, observe the stain residue
I would suggest that you also add a level of seriousness by taking notes on the concentration levels of dissolved chemicals and by using a microscope during inspection of results. By chemicals I mean whatever you can find in your house: salt, sugar, wine, food coloring, etc. Kids can easily follow the logic of this experiment and come up with ideas how to modify it. Also, encourage kids to make notes (see our older story about this).


Exposing the secrets of drying droplets
Explanation of the ring-shaped stains requires several steps. Frist, we start with the geometry of receding evaporating droplet. A small droplet takes a spherical shape thanks to the surface tension force dominating over gravity. We discussed why this happens in one of our previous newsletters, but a deep thorough understanding of surface tension in droplets is quite complicated and still investigated by scientists.
One particularly complicated and difficult part of a droplet is the contact rim where three types of matter interact: liquid droplet, solid ground surface (substrate), and surrounding air filled by evaporating liquid. It turns out that droplets often prefer to keep this contact rim stationary even when they lose their volume because of evaporation. This if the first step in this explanation.

The second step connects us to the story of how raindrops are formed in a cloud. 150 years ago, Lord Kelvin realized that the curvature of liquid surface influences its evaporation. A curved surface reduces the number of bonds that the interior molecules can form with the surface molecules. Hence, it is easier for molecules on a curved surface to escape the liquid than ones at a flat surface. The greater the curvature, the greater rate of evaporation.
The equation describing this connection between evaporation and surface curvature is named Kelvin’s equation after him. Surprisingly, it was shown recently that this equation works even on the scale of a few water molecules thick layers. When applied to the physics of water droplets formation out of pure water vapour, the equation shows that raindrops are very unlikely to form. Therefore raindrops require some tiny dust particles as nucleation points for condensation.

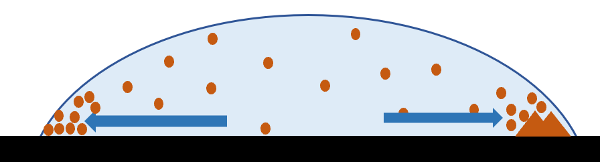
If you look now at our drying droplet, you can notice that evaporation should not be equally fast all over the surface. The contact rim is a place where the surface molecules are exposed to the most extreme curvature and, therefore, to faster evaporation. The droplet now finds itself in a situation where the liquid is quickly disappearing from the surface close to the contact rim, while this rim is stubbornly pinned to the substrate. This creates a suction that moves the liquid out of the droplet’s interior and towards the contact rim.

The stage is now set for the ring stain. The microflow takes the molecules or particles of stuff dissolved in the droplet and concentrates them at the contact rim. Since the liquid can sustain only a limited amount of this material, it starts to deposit on the substrate. This forms either a ring of residue or crystals that start to grow.
This might seem like a complicated chain of events, but this is the simplest scenario among all the effects that can exists within a drying droplet. In the next figure we list some processes that can be initiated by changing details of this experiment. The list of parameters affecting a droplet is quite long and you can easily start exploring what happens under different atmospheric pressures and humidity, air or droplet or substrate temperatures, substrates, dissolved particles properties and concentrations, multiple dissolved compounds, etc. It is easy to see now why this field of research is so prolific.

Rings of crystals
In our experiment we played with dissolving table salt (NaCl), magnesium sulphate (MgSO₄), and copper(II) sulphate (CuSO₄) in various concentrations (those sulphates are available in garden supply stores or online). When droplets dried out, crystals remained in various configurations that are fun to explore under a microscope. The main feature is that crystals tend to form a ring, but at one point they grow too big for the droplet to maintain their growth and then they appear within the ring, too.
There is an interesting story about the table salt crystals that are formed in this way. Table salt crystallizes into a cubical shape, but in this case, it wants to grow into a pyramid (half of a cube). However, growth at the crystal edges is faster than in the center, which produces a special type of crystals called hopper crystal. Such crystals have hollowed interior, and the way how to produce them in large quantities is a trade secret of some food producers.
In our case, hopper NaCl crystals are flat, and it turns out that crystallization patterns of evaporating water drops containing dissolved salts is an active filed of research. They were even grown onboard ISS under microgravity conditions. As you can see, there is a plenty of room for exploration using simple drying droplets. Just remember – taking notes is a very important part of STEM education.





