Hidden worlds in a speck of light
A simple home-made gadget can open a window into the language of atoms and molecules.
Throughout human history, people have admired rainbows. They understood that such beauty must also have a deeper meaning. The ancient Greeks were trying to understand how a rainbow is formed, and various philosophers after that searched for its secret for centuries. It took time to abandon the mystical concept of rainbows, such as the covenant between God and people, described in the Bible, Genesis 9:13, that God will never to unleash another flood.
While the pure geometrical aspect of light path through raindrops had been established already in the Middle Ages, the nature of colors remained a mystery. It seemed logical to assume that sunlight has no color, and that the material is coloring the light passing through it. Finally, about 350 years ago Newton discovered an amazing property of sunlight – it is made of colors! He performed a simple experiment. First, he created a rainbow using a prism, but then he used another prism to combine these colors back into the colorless sunlight.
This discovery opened a window into hidden worlds that Newton could not even dream of. The property of light to carry a rainbow within itself gives us a tool to explore the world around us, including the universe all the way to its far edge. In the centuries that followed, scientists discovered different properties of light that allowed them to understand the “language” of light in the form of the colors it carries.
In previous episodes, we touched on various aspects of this story:
why some colors don't really exist (amazing!)
rainbows are actually far more complex and much more fun than you expect
how come rainbows appear all around us, including soap bubbles, metal surfaces and on animals (and how to make a rainbow chocolate).
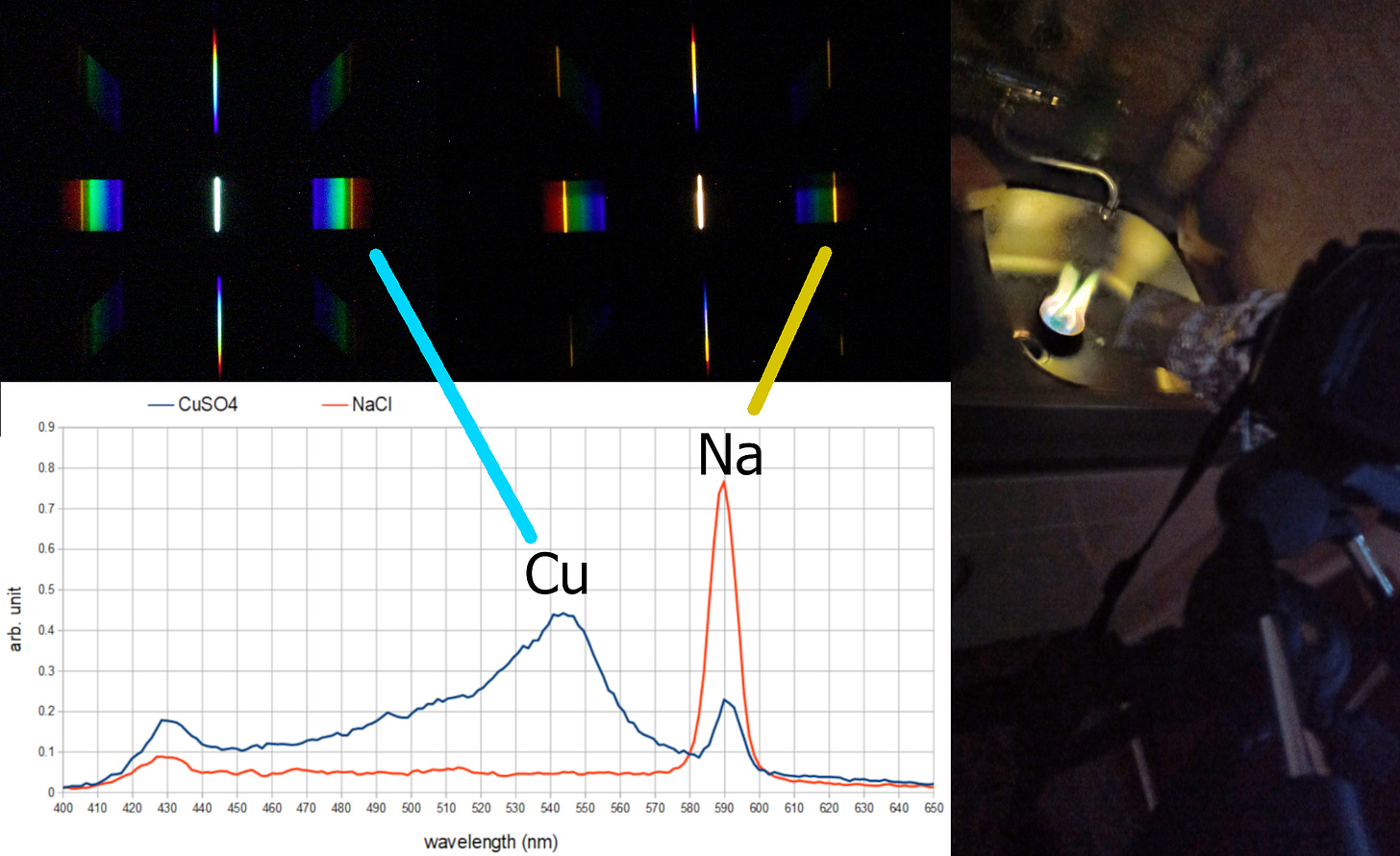
Now we focus on the language of light. This language is spoken by the matter which emits that light. Matter is made up of atoms and molecules, and they are very picky about the colors they emit and the color intensity. Scientists in the laboratory measure the specific colors that a material emits, which we call the emission spectrum. Thanks to this, if they observe light coming from an unknown source, they check whether it carries this specific spectrum. In this way, they can reveal the chemical composition of the source by deciphering the spectra hidden in the emitted light.
Similarly, the atoms and molecules in a transparent substance absorb and scatter light in a specific way that also leaves its signature in the color spectrum. So the explorers before Newton were actually right in a way. It's just that they didn't understand how this interaction between light and prism glass works.
With this approach astronomers can study the atmosphere of distant planets orbiting other stars, and chemists can determine the chemical composition of their samples. And you can get a sense of it with a very simple device that you can make together with your kids at home.
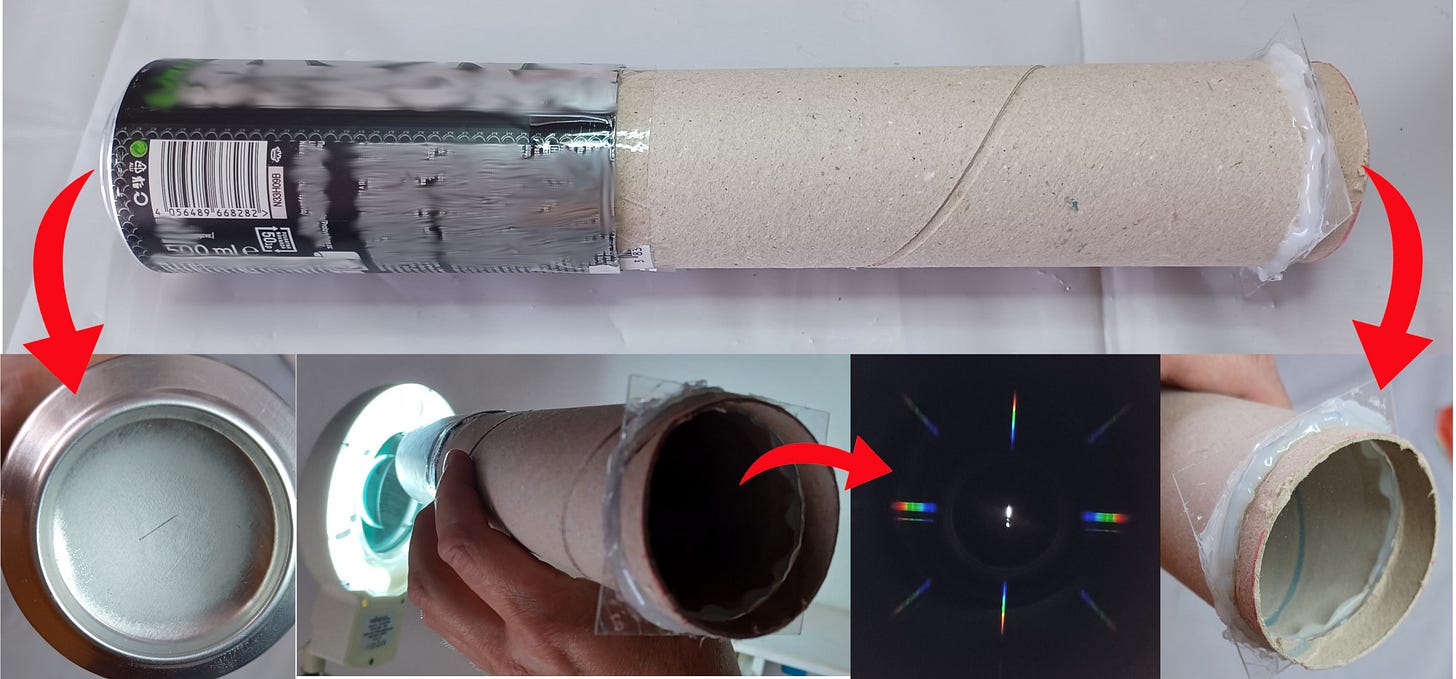
The key part you need is the diffraction foil/film (easy to buy online). Light passing through this foil is transformed into a spectrum (this is happening because of the interference effect that we described in one of our episodes).
Next you need a cardboard tube (I use a discarded kitchen paper towel tube). Attach the foil with hot glue to one end of the tube. The other end must be closed, except for a very narrow opening with sharp edges. One option is to use a razor blade to create a slit. You can also take an aluminum can, attach it to the tube and make a slit in the bottom. I used both options and it worked fine. The goal is to have a dark tube with a narrow spot of light passing through the diffraction foil. This creates rainbows which are actually the emission spectrum of the light source you are observing. You are holding a primitive version of a device called a spectrograph.
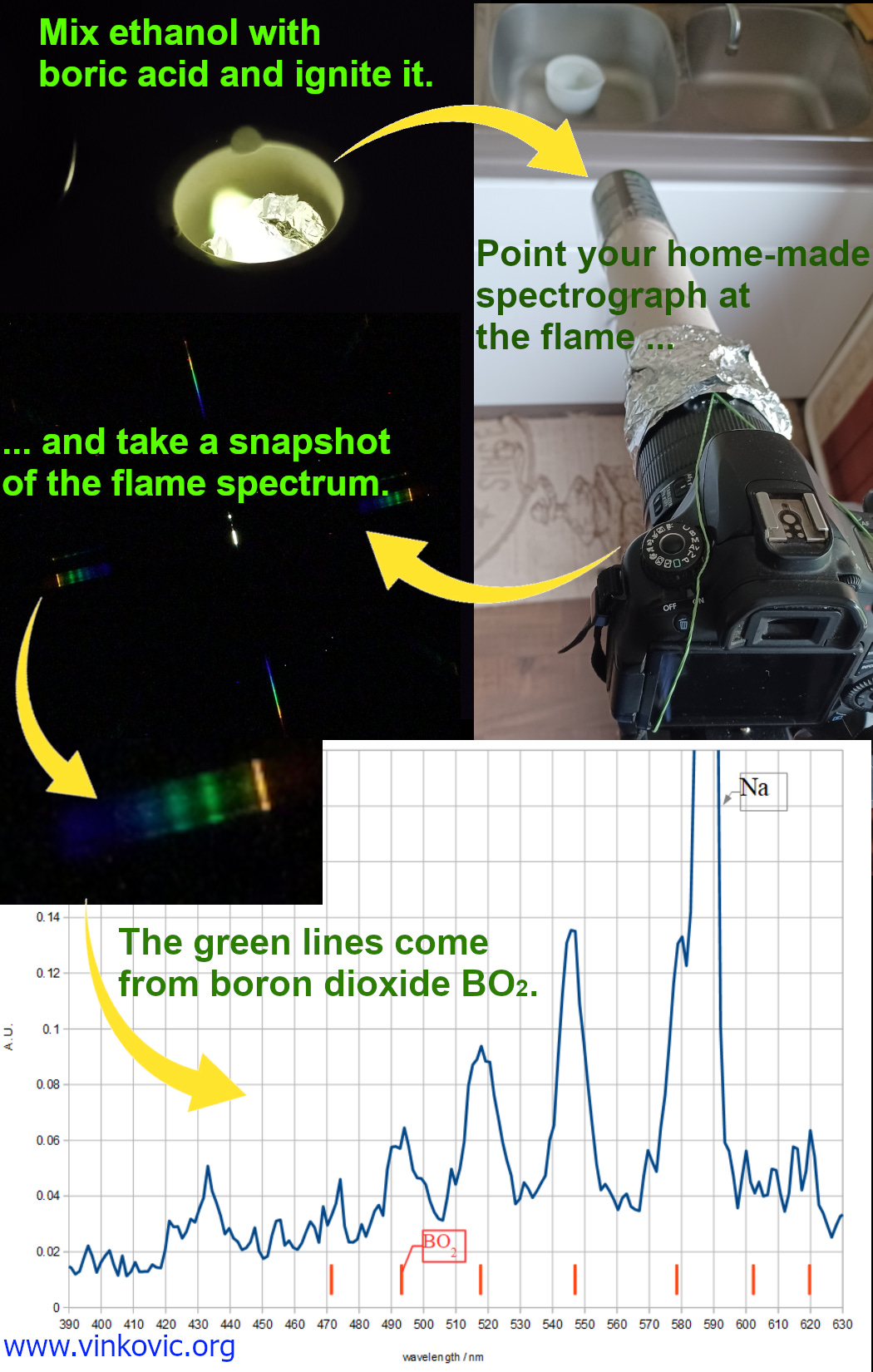
Now you can start observing the emission spectra of different light sources – the Sun, different types of light bulbs, flames, etc. We have spent many hours investigating the light that comes from flames colored with various chemicals. As an extension, we attached this spectrograph to the camera using rubber bands (we can now call this spectrometer), which opened up the possibility of measuring color intensities and comparing the spectra with data from the scientific literature (this is an ideal topic for a science fair project!).
Here are some examples of our results (we also observed the spectra of stars, but this is a story for another day):








This is very cool, never heard of diffraction foil, I need to make the same thing at home !